Home > Publications
Si M, Fu J, Fang M, Lu Y, Huang J, Li H, Wang P, Liao M, Zhu J, Li P, Zhong W, Guo Z, Yang W, Ye Z, Hu H, Yu X. Lysosome-mediated aggregation of galactose-deficient IgA1 with transferrin receptor 1 links to IgA nephropathy. Nat Commun. 2025 Jul 1;16(1):5536. doi: 10.1038/s41467-025-60819-w. PMID: 40593574; PMCID: PMC12219878.
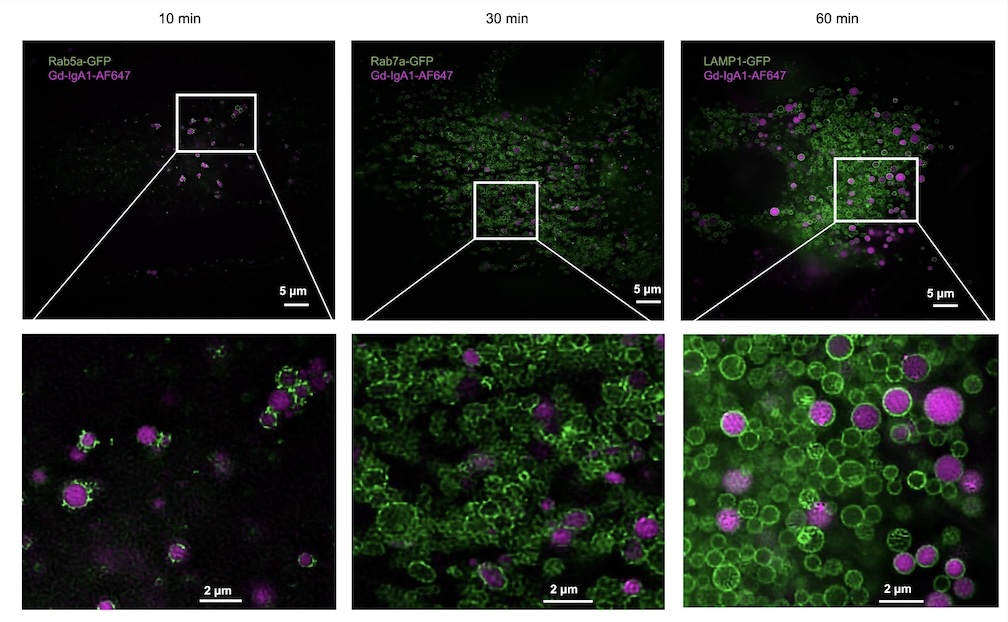
The retention of galactose-deficient IgA1 (Gd-IgA1) in the mesangium is central to IgA nephropathy (IgAN), but its intracellular fate remains unclear. Here, we show that transferrin receptor 1 (TfR1) mediates Gd-IgA1 uptake into mesangial cell lysosomes, where it forms non-digestible aggregates, disrupts lysosomal function, and triggers inflammatory responses. In renal biopsies from IgAN patients, IgA1 aggregates co-localize with TfR1 within lysosomes. In male mice, TfR1 overexpression enhanced lysosomal accumulation of Gd-IgA1, whereas TfR1 knockdown reduced it. Mechanistically, acidic pH strengthens TfR1–Gd-IgA1 binding via the galactose-deficient hinge region and residue R276. While we acknowledge that sialylation commonly found in patient-derived IgA1 might influence TfR1 binding and that other receptors, such as ASGPR, were not evaluated, our findings nonetheless reveal a lysosome-centered mechanism in IgAN and highlight receptor-mediated retention of Gd-IgA1 as a potential therapeutic target.